1 1 3 6 2 2 4 4. Why does a lithium atom.

Ions Atoms Are Neutral But When An Atom Gains Or Loses An Electron It Becomes An Ion Ions Can Be Positive Or Negative Ppt Download
1560 students attemted this.

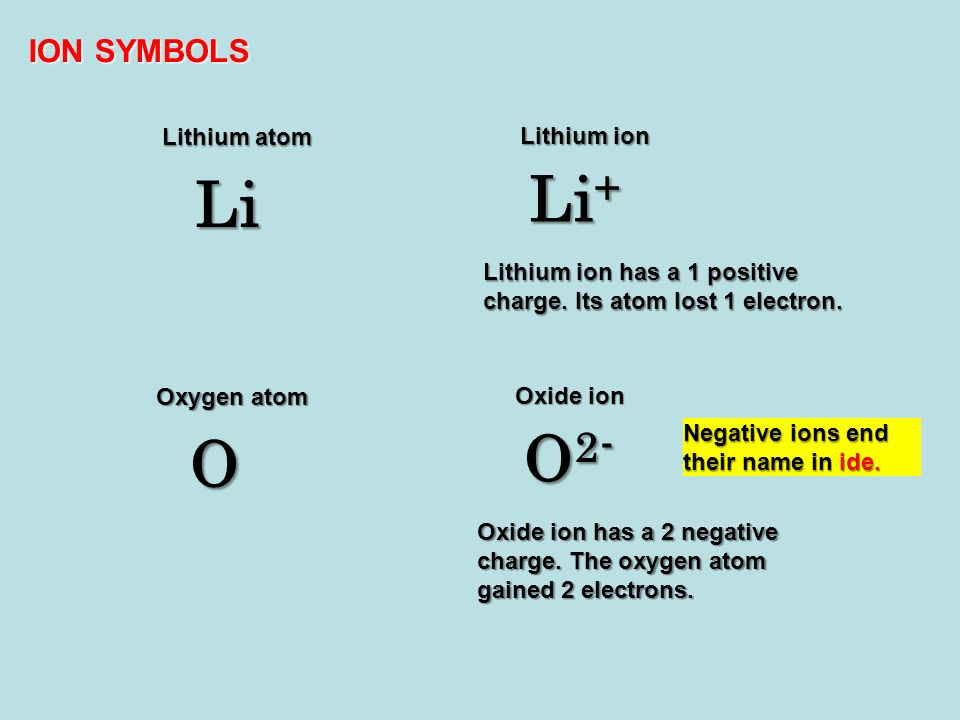
. And when an atom loses an electron it becomes a positive ion because electrons are negative charged. When an atom loses an electron the atom becomes a positively charged ion. When an atom of lithium loses an electron the atom becomes a General.
12 When an atom of lithium loses an electron the atom becomes a 1 negative ion with a radius smaller than the radius of the atom 2 negative ion with a radius larger than. When an atom of lithium loses an electron the atom becomes a When an atom loses an electron it becomes a positive ionWhen an atom of lithium loses an electron and becomes an ion it has one less electron shell so its radius is smaller than the radius of the atom. When helium atom loses one electron it becomes positive ion with 1 charge.
11 What is the total number of electron pairs shared between the two atoms in an O 2 molecule. Oxygen has a double bond so 2pairs of electrons. When the metal loses an electron it changes from a metal to an ion.
A lithium atom has no electrical charge while a lithium cation has a charge of 1 because it loses an electron. When an atom of lithium loses an electron the atom becomes. This is because now the.
If lithium loses one electron then it is said to have a charge of 1 if 2 electrons lost then the charge would be 2 and if all the electrons are lost then charge would be 3. Moreover when it loses two electrons its become positive ion with 2 charge on it. Lithium has 3 electrons 2 in inner shell and 1 in outer shell.
Remember that when an atom loses electron. Because Lithium atom has has 3 electrons and if it loses an electron itll lose the electron in its 2s shell which means that atom will become smaller in size. When an atom of lithium loses an electron the atom becomes a.
Thus there are three electrons in a lithium atom. When a helium atom loses electrons it get positive charge because the number of protons in the helium atom becomes greater than the number of electrons. When lithium loses an electron from its outermost orbit it forms the positive ion called lithium ion Li.
Positive ion with a radius smaller than the radius atom loses an electron means less Identify the element in the table classified as a. When an atom of lithium loses an electron the atom becomes a positive ion with a radius smaller than the radius of the atom. Also know what charge do lithium ions have.

Ions Ionic Bonding O Level Chemistry Notes
The Periodic Table Ions Ppt Download

Ionisation Energy And The Periodic Table Siyavula Textbooks Grade 10 Physical Science Openstax Cnx
0 Comments